

approach & pipeline
DEFINED TARGETS.
BOUNDLESS IMPACT.
Dianthus Therapeutics is developing next-generation therapies with proven mechanisms of action and best-in-class potential to transform the treatment of severe autoimmune diseases.
OUR INNOVATION
Both of our clinical candidates, claseprubart and DNTH212, demonstrate strong pipeline-in-a-product potential, underpinned by validated mechanisms of action across a range of severe autoimmune indications.
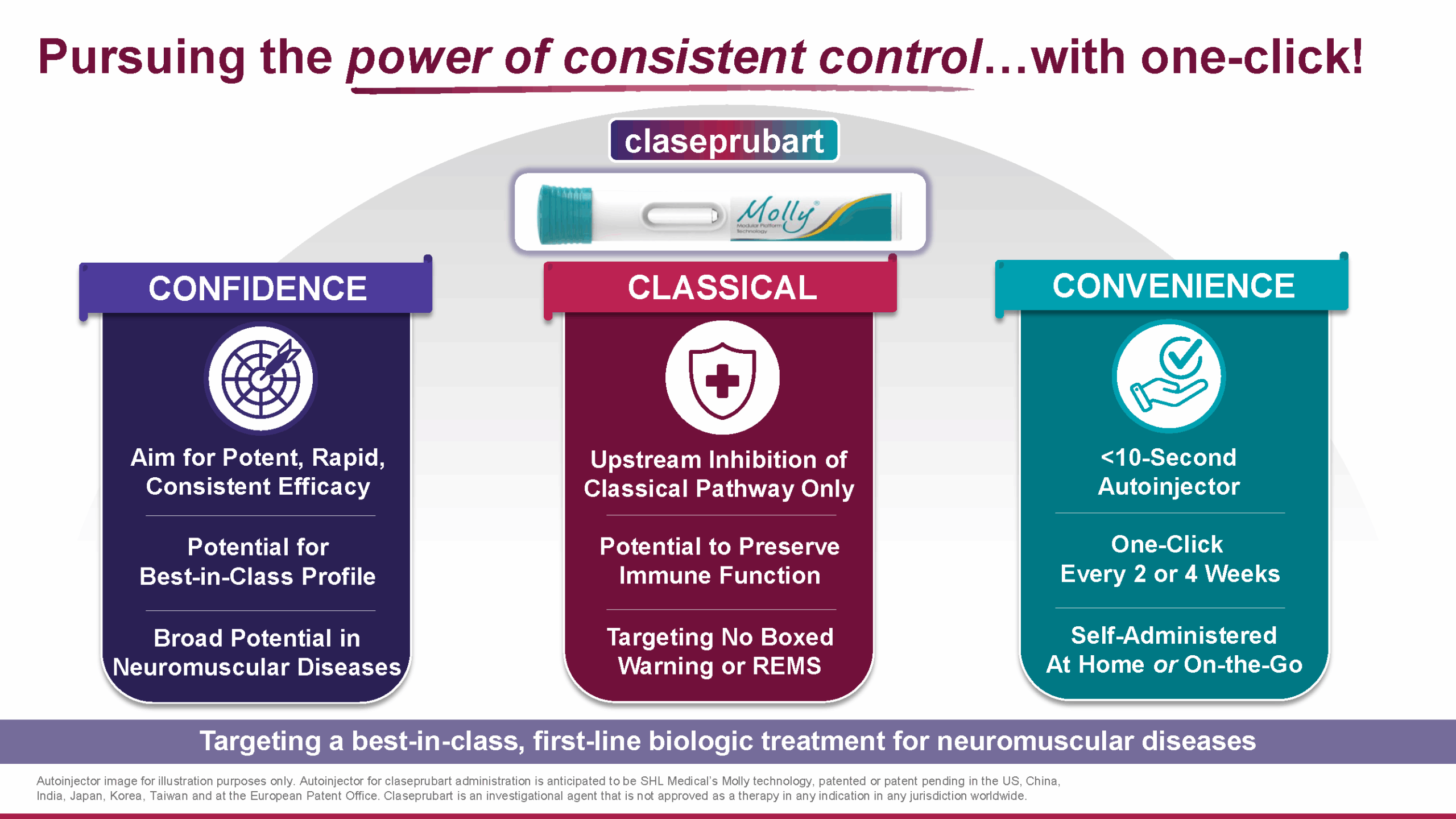
Our lead candidate, claseprubart (DNTH103) is a potentially best-in-class complement inhibitor purposefully engineered with extended half-life, improved potency, and high selectivity for only the active C1s complement protein that drives disease pathology – enabling less frequent and more convenient self-administered subcutaneous injections.
DNTH212 is a first and potentially best-in-class, long-acting bifunctional fusion protein designed to reduce Type 1 interferon production, while simultaneously inhibiting BAFF/APRIL signaling to suppress B cell function.
Addressing both the disease and treatment burdens that disrupt the lives of patients.
OUR FOCUS
Claseprubart
Claseprubart is an investigational, long-acting monoclonal antibody engineered to potently and selectively inhibit the active form of C1s, a clinically validated target in the classical complement pathway.
As the classical pathway plays a significant role in disease pathology across a range of neuromuscular disorders, claseprubart holds the potential to be a pipeline in a product – beginning with generalized Myasthenia Gravis (gMG), Multifocal Motor Neuropathy and Chronic Inflammatory Demyelinating Polyneuropathy.
Engineered with YTE half-life extension technology, claseprubart is intended to be the first subcutaneous complement therapy that can be self-administered as infrequently as once every two weeks for patients with severe, classical pathway-driven autoimmune disorders.

DNTH212
DNTH212 is an investigational, first and potentially best-in-class, extended half-life bifunctional fusion protein targeting plasmacytoid dendritic cell (pDC) BDCA2 to reduce Type 1 interferon production, while simultaneously inhibiting BAFF/APRIL to suppress B cell function.
By targeting the innate and adaptive immune systems via two clinically validated pathways that are known drivers of autoimmune disease pathogenesis, this complementary and differentiated approach has the potential to address multiple autoimmune indications with improved outcomes, representing a pipeline-in-a-product opportunity.
Engineered with YTE half-life extension technology, DNTH212 is intended to be self-administered as infrequently as once every four weeks for patients with severe autoimmune disorders.

BUILDING AN AUTOIMMUNE FRANCHISE WITH CLASEPRUBART and DNTH212
Dianthus is building a leading autoimmune franchise.
The Company expects to initiate a Phase 3 trial of claseprubart in gMG in 2026, conduct an interim responder analysis of the Phase 3 CAPTIVATE trial of claseprubart in CIDP in Q2’26 and report top-line data from the Phase 2 MoMeNtum trial of claseprubart in MMN in 2H’26.
A two-part Phase 1 study in China in healthy volunteers (Part A) and patients with systemic lupus erythematosus (Part B) of DNTH212 is ongoing, with top-line results in healthy volunteers expected in 2H’26.
PIPELINE
Claseprubart – subcutaneous active C1s antibody
Ongoing Discovery
*Rights outside of greater China licensed from Nanjing Leads Biolabs Co., Ltd.; being developed by Leads in China as LBL-047.




