

approach & pipeline
DEFINED TARGETS.
BOUNDLESS IMPACT.
Dianthus Therapeutics is harnessing the power of selectivity to advance next-generation complement therapies to treat severe autoimmune diseases.
OUR INNOVATION
Our lead antibody, DNTH103, is purposefully engineered with extended half-life, improved potency, and high selectivity for only the active C1s complement protein that drives disease pathology – enabling less frequent and more convenient self-administered subcutaneous injections.
Addressing both the disease and treatment burdens that disrupt the lives of patients.
OUR FOCUS
DNTH103 is an investigational, long-acting monoclonal antibody engineered to potently and selectively inhibit the active form of C1s, a clinically validated target in the classical complement pathway.
As the classical pathway plays a significant role in disease pathology across a range of neuromuscular disorders, DNTH103 holds the potential to be a pipeline in a product – beginning with generalized Myasthenia Gravis (gMG), Multifocal Motor Neuropathy and Chronic Inflammatory Demyelinating Polyneuropathy.
Engineered with YTE half-life extension technology, DNTH103 is intended to be the first subcutaneous complement therapy that can be self-administered as infrequently as once every two weeks for patients with severe, classical pathway-driven autoimmune disorders.
So patients can live healthier lives to their fullest potential.
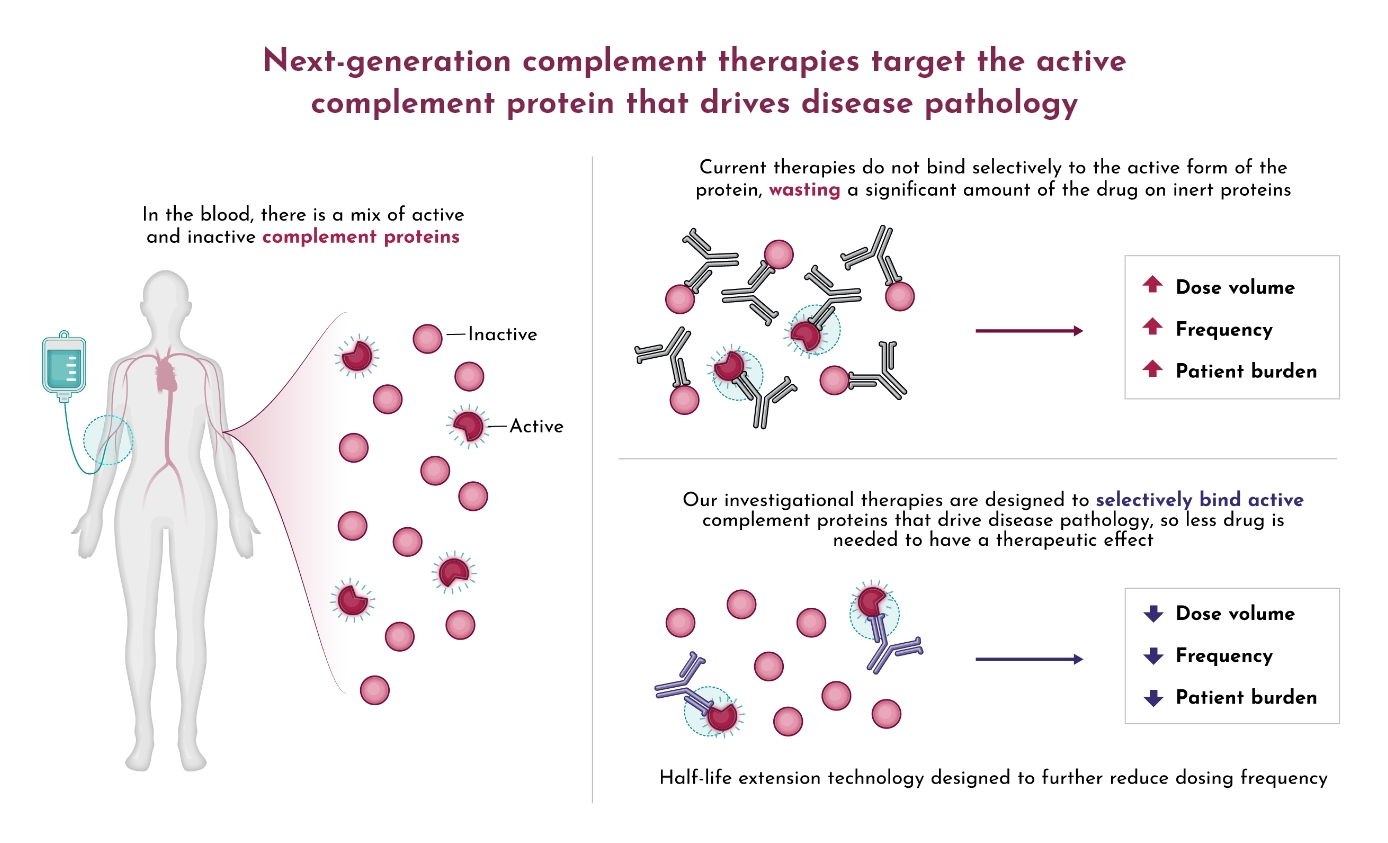
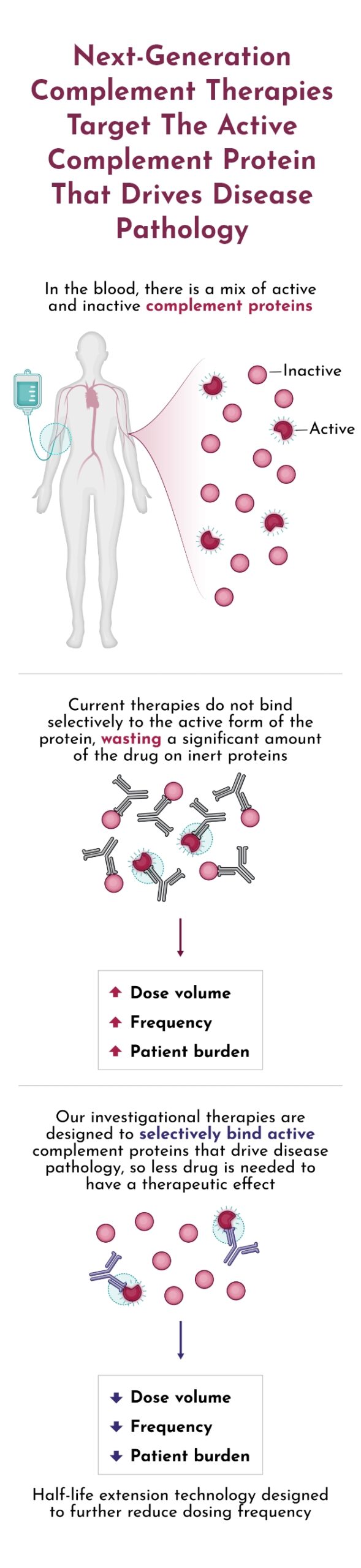

BUILDING A NEUROMUSCULAR FRANCHISE WITH DNTH103
Positive top-line Phase 1 data for DNTH103 from 60 healthy volunteers across eight single and multiple-ascending dose cohorts validate DNTH103’s potent classical pathway inhibition with an approximate 60-day extended half-life and a potentially differentiated safety profile. Dianthus is building a neuromuscular franchise with ongoing trials of DNTH103 in generalized Myasthenia Gravis, Multifocal Motor Neuropathy, and Chronic Inflammatory Demyelinating Polyneuropathy.
PIPELINE
DNTH103 – subcutaneous active C1s antibody
Ongoing Discovery




